
Jena, Germany, July 18, 2019 – InflaRx (Nasdaq: IFRX), a clinical-stage biopharmaceutical company developing anti-inflammatory therapeutics by targeting the complement system, today reported additional in-depth analysis of the completed double blind, placebo controlled part of the ongoing international SHINE Phase IIb study to supplement InflaRx’s top line disclosure of results reported on June 5, 2019. The purpose of this study is to investigate the safety and efficacy of IFX-1, a first-in-class anti-human complement factor C5a monoclonal antibody, in patients suffering from moderate to severe Hidradenitis Suppurativa (HS), a painful and debilitating chronic inflammatory skin disease with limited treatment options.
Prof. Niels C. Riedemann, CEO and Founder of InflaRx, commented: “While we were very disappointed about missing our primary endpoint in the SHINE study, our in depth post-hoc data analysis suggests a robust anti-inflammatory activity in the high dose IFX-1 treatment group across numerous efficacy measures which were not reflected by the HiSCR. The company has made significant efforts to research the available data set and has engaged with US and European experts to assist in the analysis and interpretation of these findings. Based on this, our team continues to work on the development of IFX-1 in HS. We look forward to discussions with the regulatory authorities upon completion of the open label extension portion of the ongoing SHINE study”, he added.
The randomized, double-blind, placebo-controlled, multicenter study, which enrolled a total of 179 patients in four active dose arms and a placebo arm at over 40 sites in North America and Europe, recently reported a failure to achieve its primary endpoint, a dose dependent drug effect on Hidradenitis Suppurativa Clinical Response Score (HiSCR). In order to be a responder in this binary score, the total body count of abscesses and inflammatory nodules (AN count) must be reduced by at least 50% while the abscesses and draining fistula count may not accede baseline counts. The score thus does not account for any reduction in draining fistulas. The placebo arm demonstrated an unusually high HiSCR response rate at week 16 of 47.2%, when compared to earlier reported larger studies (Pioneer 1: 26% / Pioneer 2: 27.6%, both at week 12). An in-depth patient by patient data analysis revealed a very high variability of the HiSCR responses in all trial groups, which was strongly affected by a large individual fluctuation of the AN count.
The inclusion and exclusion criteria of the SHINE study were nearly identical to the ones used in an earlier published trial, the PIONEER II study, and the baseline characteristics of the placebo group in the SHINE study were comparable to those of the PIONEER II study with a good distribution across all dose groups. This accounts especially for the median AN count, median draining fistula count, gender distribution, Hurley Stage II and III distribution and others. Despite various additional efforts including post-hoc sensitivity analysis on parameters like differences in HiSCR responder rates in patients with low or high AN count at baseline, in Hurley II versus III patients, in severe versus moderately diseased patients, as well as clinical trial-site analysis, and several others, the profound difference in the HiSCR response rates between these two trials could not be explained by the results of these analyses. Importantly, no underlying trial conduct problem to account for the high placebo HiSCR rate could be detected.
InflaRx conducted an in-depth efficacy analysis (secondary endpoints as well as additional post-hoc analysis) upon receipt of the full data set of the first 16 weeks of the SHINE study and, consecutively, consulted with key opinion leaders (“KOL”) and members of its Scientific Steering Committee in the United States, Canada and Europe. This analysis revealed multiple efficacy signals for the high dose group when compared to the placebo group:
At week 16, there was a statistically significant reduction of draining fistulas (“DF”) relative to baseline in the high dose IFX-1 group when compared to placebo (Figure 1 – relating to all patients with at least 1DF at baseline).
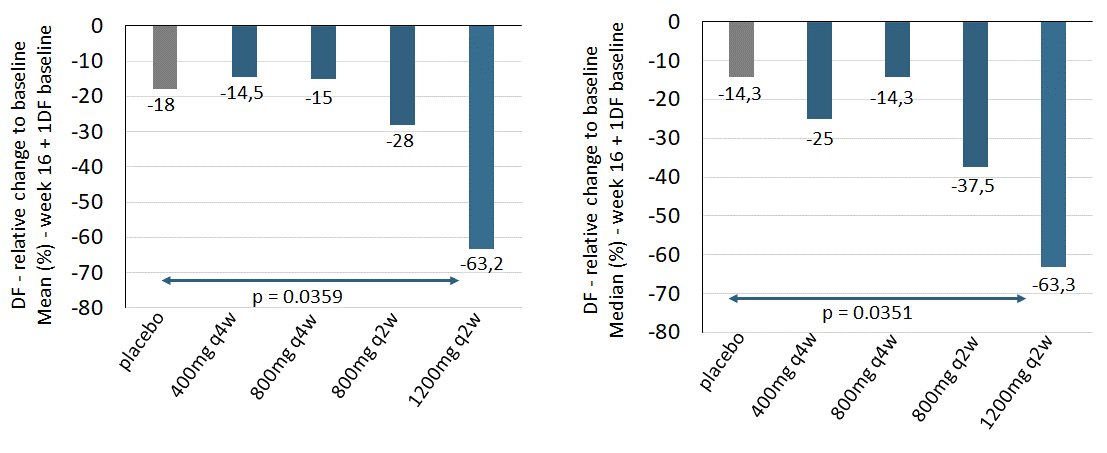
Figure 1: Draining Fistula (DF) reduction relative to baseline at week 16 (left: Mean, right: Median) in all patients with at least 1 draining fistula at baseline. For mean comparisons and the p-value of high dose versus placebo, an ANCOVA model adjusted for DF and Hurley stage at baseline was calculated. The p-value for the median comparison of high dose versus placebo was based on the Wilcoxon rank-sum test. Complete case analysis, no imputation of missing values.
This reduction in DF was visible as early as 2 weeks after induction of high dose IFX-1 therapy and consistent over time with the strongest observed reductions seen at weeks 6, 8 and 16 (Figure 2). A temporary weakening of the strong reduction was observed between weeks 10 to 14 which could not be explained by pharmacokinetic or pharmacodynamic parameters. The strong relative reduction of draining fistulas observed in the SHINE trial was consistent with earlier findings in the open label Phase IIa study (manuscript under revision for publication).
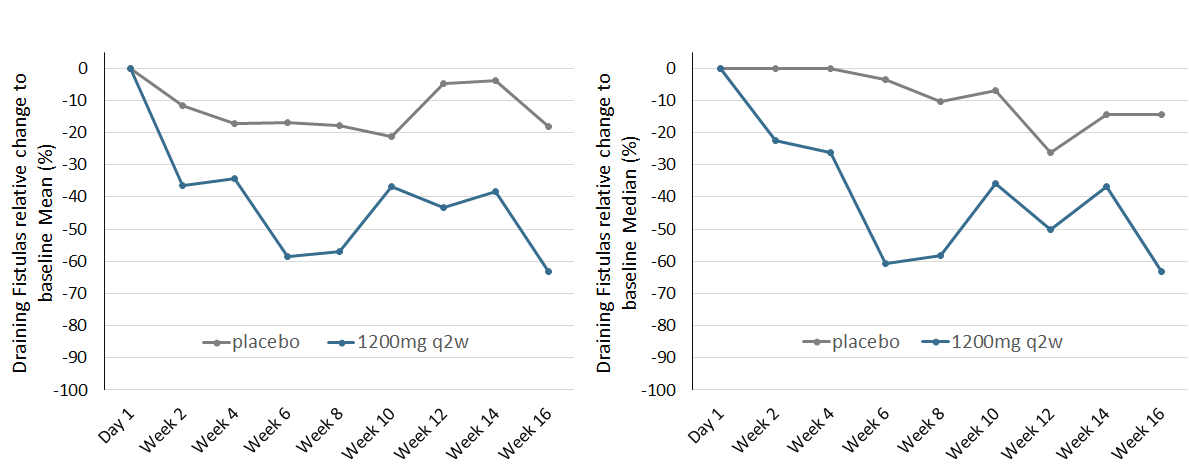
Figure 2: Draining Fistula (DF) reduction relative to baseline per visit (left: Mean, right: Median) until week 16 for placebo and the high dose group (IFX-1 1200mg q2w) in all patients with at least one DF at baseline. For mean comparisons of high dose versus placebo, an ANCOVA model adjusted for DF and Hurley stage at baseline was calculated. Complete case analysis, no imputation of missing values.
As indicated in the initial press release from June 5, 2019, IFX-1 therapy reduced the AN count at week 16 relative to baseline with a trend to a dose dependent effect. Further analysis showed that high dose IFX-1 therapy reduced abscesses and inflammatory nodule counts over time (Figure 3):
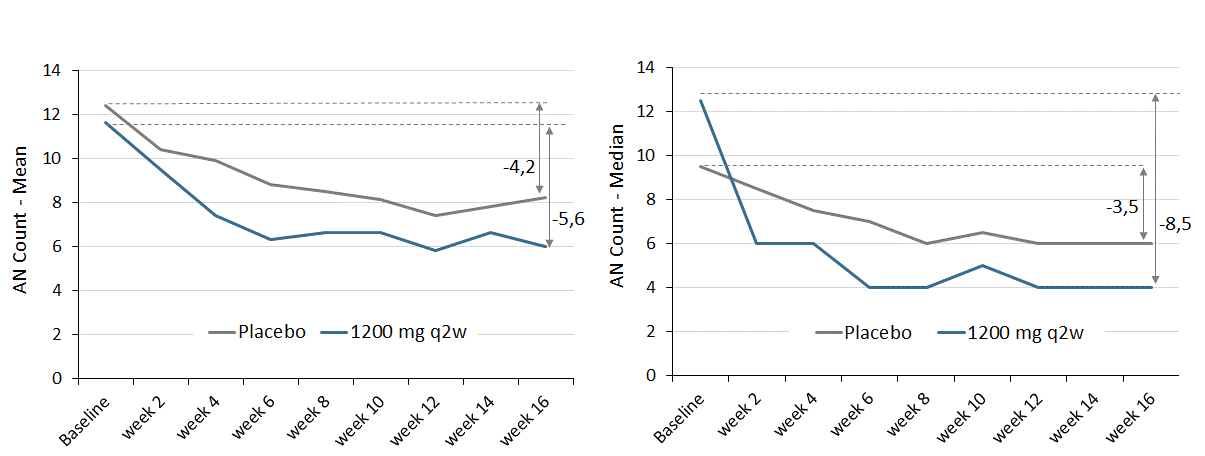
Figure 3: AN count per patient visit (left: Mean, right: Median) until week 16 for placebo and high dose group (IFX-1 1200mg q2w). Complete case analysis, no imputation of missing values.
As a result of discussions with both, the Scientific Steering Committee, and leading KOLs, InflaRx conducted an additional post-hoc analysis utilizing the International Hidradenitis Suppurativa Severity Score System (“IHS-4”), which is scoring the patient according to all inflammatory lesions in contrast to the HiSCR. In this compounded score, each inflammatory nodule is counted with 1 point, each abscess with 2 points and each draining fistula with 4 points. Patients scoring with 0-4 are considered mild, with 5-10 moderate and with a score of 11 or higher as severe. Applying the IHS-4 score, there was a statistically significant relative change to baseline at the end of the treatment period on week 16 (Figure 4) when comparing the high dose IFX-1 treated group to placebo group. A trend to a dose response across IFX-1 treated groups when compared to placebo could be detected.
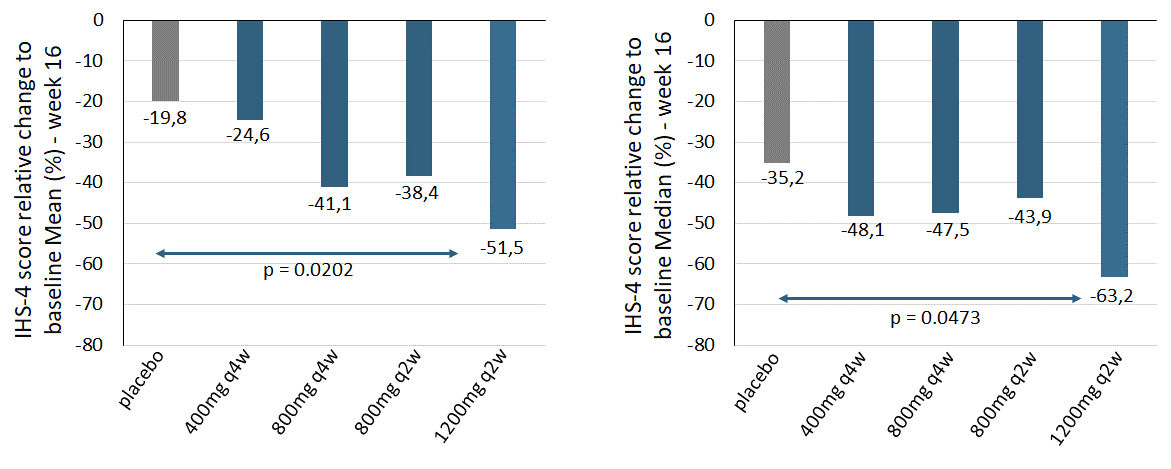
Figure 4: IHS4 score reduction relative to baseline at week 16 (left: Mean, right: Median) for placebo and the high dose group (IFX-1 1200mg q2w). For mean comparisons and the p-value of high dose versus placebo, an ANCOVA model adjusted for IHS4 and Hurley stage at baseline was calculated. The p-value for the median comparison of high dose versus placebo is based on the Wilcoxon rank-sum test. Complete case analysis, no imputation of missing values.
The relative change over time of the IHS-4 score showed similar patterns in the placebo and high dose group when compared to the relative change in DF with the strongest reductions at week 6, 8 and 16 together with a temporary weakening of the signal during week 10 to 14. Efficacy signals were also detected in the high dose group for the Hidradenitis Suppurativa Physician Global Assessment Score (“HS-PGA”), which scores patients’ severity in six categories: clear, minimal, mild, moderate, severe and very severe according to the amount and quality of inflammatory lesions. IFX-1 treatment resulted in a more pronounced shift of severe and very severe patients into groups of lower severity when compared to the placebo group. This difference was most prominent on week 8 and week 16. Patients shifting to the category clear disease were only detected in IFX-1 treated groups but not in the placebo group at week 16.
Pharmacokinetic and pharmacodynamic (“PK/PD”) analysis demonstrated a dose dependent suppression of C5a and IFX-1 levels and suggested that the high dose group (1200mg q2w) offered a superior control over C5a levels when compared to the other dosing groups. IFX-1 treatment in all dose groups resulted in sustained CH50 levels, as sign of an intact activation of the membrane attack complex confirming earlier findings. The company is running additional population PK analysis and statistical models to estimate tissue PK, using all available data, to guide possible future dosing and to estimate tissue penetration of IFX-1 in HS.
The detection rate of confirmed anti-drug antibodies (“ADA”) in the first 16 weeks of the SHINE trial was overall low (<10% including pre-dose findings) for patients in both, the placebo group and the combined IFX-1 treated groups.
The company is continuing the open label extension part of the SHINE trial with the last patient dosing being expected for end of August 2019. Additional data will be analyzed as it becomes available.
Conference call details:
Date: Friday, 19 July 2019
Time: 8 am EDT, 2 pm CEST
From the US: 877-407-0789
From outside the US: +1 201-689-8562
Conference ID: 13692883
To join the audio-only webcast, register here: http://public.viavid.com/index.php?id=135498
Please dial in 10 minutes before the call to register.
About IFX-1:
IFX-1 is a first-in-class monoclonal anti-human complement factor C5a antibody, which highly and effectively blocks the biological activity of C5a and demonstrates high selectivity towards its target in human blood. Thus, IFX-1 leaves the formation of the membrane attack complex (C5b-9) intact as an important defense mechanism, which is not the case for molecules blocking the cleavage of C5. IFX-1 has been demonstrated to control the inflammatory response driven tissue and organ damage by specifically blocking C5a as a key “amplifier” of this response in pre-clinical studies. IFX-1 is believed to be the first monoclonal anti-C5a antibody introduced into clinical development and has, to date, successfully completed three clinical Phase II studies. More than 150 people have been treated with IFX-1 in these completed clinical trials, and the antibody has been shown to be well tolerated. IFX-1 is currently being developed for various inflammatory indications, including Hidradenitis Suppurativa, ANCA-associated vasculitis and Pyoderma Gangraenosum.
About InflaRx N.V.:
InflaRx (Nasdaq: IFRX) is a clinical-stage biopharmaceutical company focused on applying its proprietary anti-C5a technology to discover and develop first-in-class, potent and specific inhibitors of C5a. Complement C5a is a powerful inflammatory mediator involved in the progression of a wide variety of autoimmune and other inflammatory diseases. InflaRx was founded in 2007 and the group has offices and subsidiaries in Jena and Munich, Germany, as well as Ann Arbor, MI and New York, NY, USA. For further information please visit www.inflarx.com.
Contacts:
Investor Relations
InflaRx N.V.
Jordan Silverstein
Head of Corporate Development and Strategy
jordan.silverstein[at]inflarx.de
+1 917 837 1709
Media Relations
MC Services AG
Katja Arnold, Laurie Doyle, Andreas Jungfer
+49 89 210 2280
FORWARD-LOOKING STATEMENTS
This press release contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “estimate,” “predict,” “potential” or “continue” and similar expressions. Forward-looking statements appear in a number of places throughout this release and may include statements regarding our intentions, beliefs, projections, outlook, analyses and current expectations concerning, among other things, our ongoing and planned preclinical development and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our intellectual property position, our ability to develop commercial functions, expectations regarding clinical trial data, our results of operations, cash needs, financial condition, liquidity, prospects, future transactions, growth and strategies, the industry in which we operate, the trends that may affect the industry or us and the risks uncertainties and other factors described under the heading “Risk Factors” in InflaRx’s periodic filings with the Securities and Exchange Commission. These statements speak only as of the date of this press release and involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements, and we assume no obligation to update these forward-looking statements, even if new information becomes available in the future, except as required by law.