Loading
Loading
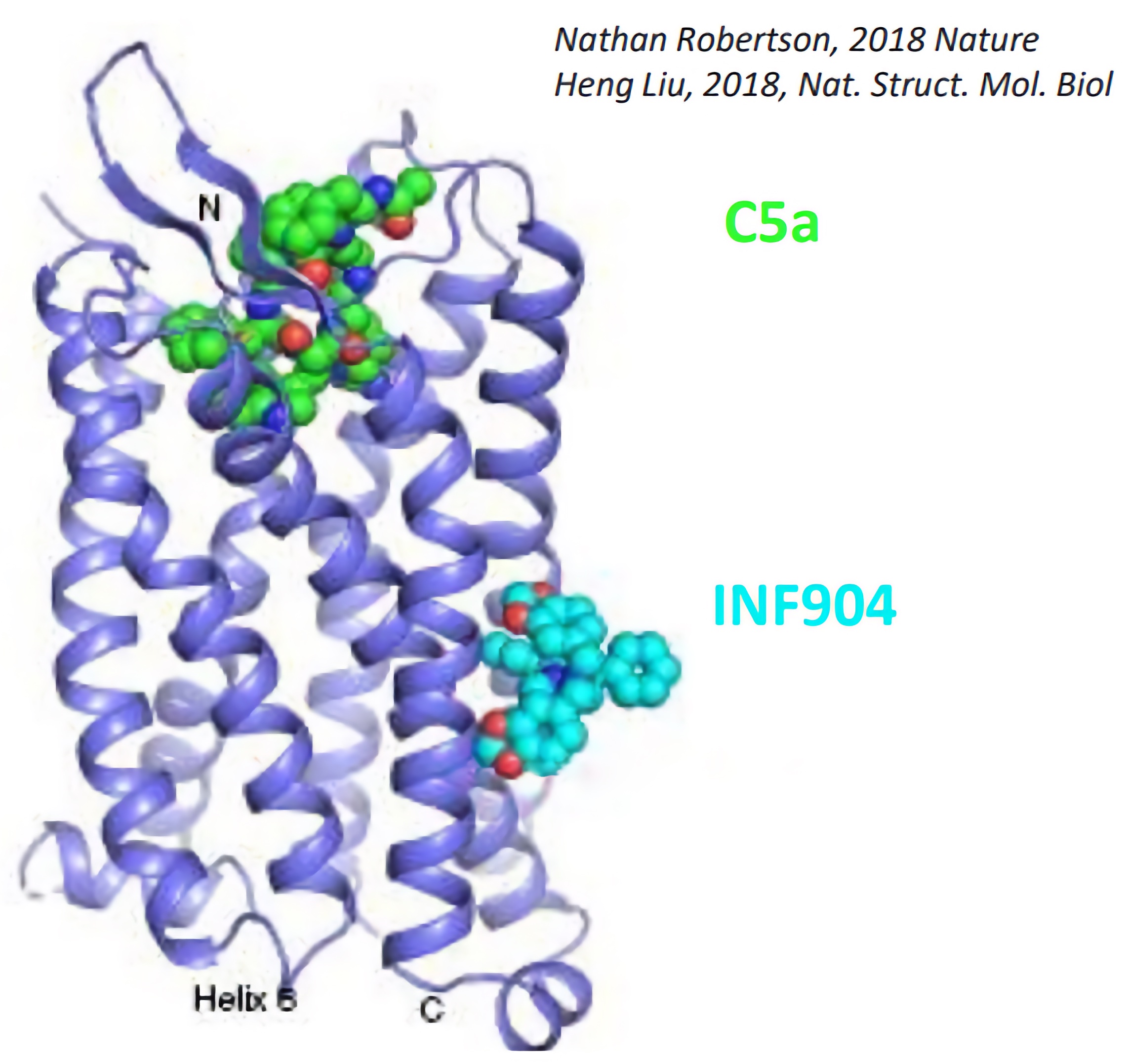
INF904 is a potentially best-in-class oral, low molecular weight drug that targets C5aR with high affinity and selectivity; and binds to an allosteric site on C5aR1 within the lipid bilayer, antagonizing the binding of C5a to block induction of neutrophil activation.
As a “pipeline-in-a-product” InflaRx is addressing complement-mediated, immune and inflammatory conditions with INF904, with an initial focus on chronic spontaneous urticaria (CSU) and hidradenitis suppurativa (HS). InflaRx is also currently conducting a Phase 2a trial, and additional pre-clinical studies with INF904, including chronic toxicology studies, as part of its effort to enable longer-term dosing of INF904 in future clinical trials. While InflaRx intends to focus its resources on its immediate goals in CSU and HS, the company continues to assess the value of pursuing additional immuno-inflammation applications in areas such as nephrology, neurology and hematology, via potential future collaborations with partners.
In preclinical studies, INF904 showed higher plasma exposure in animals, including non-human primates, and improved inhibitory activity in a hamster neutropenia model compared to the marketed C5aR inhibitor. Further, in contrast to the marketed C5aR inhibitor, in vitro experiments demonstrated that INF904 has minimal inhibition of the cytochrome P450 3A4/5 (CYP3A4/5) enzymes, which play an important role in the metabolism of a variety of metabolites and drugs, including glucocorticoids. Anti-inflammatory therapeutic effects in several preclinical disease models were also demonstrated by INF904 as well.
Reported results from a first-in-human study demonstrated that INF904 has best-in-class potential, is well tolerated in treated subjects and exhibits no safety signals of concern in single doses ranging from 3 mg to 240 mg or multiple doses ranging from 30 mg once per day (QD) to 90 mg twice per day (BID) for 14 days. Pharmacokinetic / pharmacodynamic data showed INF904 provided ≥90% blockade of C5a-induced neutrophil activation achieved over the 14-day dosing period. In addition, compared to the marketed C5aR inhibitor, INF904 showed ~3-fold higher Cmax and ~10-fold higher AUClast (at comparable dosing levels) and faster achievement of therapeutic exposures with a broad therapeutic index (at BID and QD dosing).
InflaRx is pursuing two initial immuno-dermatology indications with INF904 in a single Phase 2a basket trial that began at the end of 2024. The trial is a multi-center, open-label study expected to include a total of 75 patients with moderate-to-severe CSU and moderate-to-severe HS, and will evaluate multiple INF904 dosing regimens over 4 weeks of treatment to generate additional safety and pharmacokinetic (PK) data and to provide signs of clinical benefit. This basket study is utilizing a commercially viable formulation of INF904, providing a range of drug exposures comparable to the reported levels in the Phase 1 study. After the 4-week treatment period, patients will be followed for an additional 4 weeks.
In the CSU group, a total of 45 patients will be dosed in three study arms. Patients in Study Arms 1 and 2 will be randomized at a 1:1 ratio to 2 doses of INF904 at 60 mg or 120 mg BID (twice daily), a range of drug exposures comparable to the Phase 1 trial. Patients in Study Arm 3 will be comprised of anti-IgE treatment non-responders and dosed at 120 mg BID. In addition to safety and PK parameters, assessed CSU efficacy measures will include change in the Urticaria Activity Score 7 (UAS7), Hives Severity Score (HSS7) and Itch Severity Score (ISS7) from baseline to the end of week 4. Responder analyses, biomarkers and Patient-Reported Outcome (PRO) endpoints related to urticaria control and quality of life will also be assessed.
In the HS group, 30 patients will be randomized at a 1:1:1 ratio to 3 doses of INF904 at 60 mg, 90 mg or 120 mg BID, a range of drug exposures comparable to the Phase 1 trial. In addition to safety and PK parameters, assessed HS efficacy measures will include change in total abscess, inflammatory nodule and draining tunnel (dT) count, HS lesions-related scores and Clinician’s Global Impression of Change (CGI-C) at 4 weeks. PRO endpoints related to HS disease control, pain and quality of life will also be assessed.
Initial data from this study are expected in the summer of 2025, with a goal of informing the design of a larger, longer-term Phase 2b study by year-end 2025.