
The complement system, also known as complement cascade, is a part of the innate immune system that enhances (complements) the ability of antibodies and phagocytic cells to clear microbes and damaged cells from an organism, promote inflammation to attract additional phagocytes and attack the pathogen's cell membrane through activation of the cell-killing membrane attack complex (MAC).
The complement system consists of about 50 proteins and protein fragments that are synthesized by the liver and circulate in the blood as inactive precursors. When stimulated by one of several triggers, proteases in the system cleave specific proteins to release cytokines and initiate an amplifying cascade of further cleavages.
Three biochemical pathways activate the complement system: a) the classical pathway which can be set in motion by antigen-antibody complexes that react with activated C1q, b) the lectin or mannose binding lectin (MBL) pathway which is initiated by either serum MBL or ficolins that recognize certain sugars on microbial surfaces and c) the alternative pathway which is activated either by the presence of foreign surfaces such as lipopolysaccharides or through C3b generated by spontaneous hydrolysis, the so-called “tick-over”.
All three pathways merge at the level of C3 which can be enzymatically cleaved / activated by C3 convertases resulting in the formation of C3a and C3b. C3b is a very important factor which directs our immune system to recognize foreign cells and particles which need to be processed by our T-cells and B-cells to generate antibodies against them. Once the C3 convertase enzyme is formed, its cleavage results in formation of the enzyme C5 convertase which activates / cleaves C5 to C5a, a potent anaphylatoxin, and C5b. The C5b then recruits and assembles C6, C7, C8 and multiple C9 molecules to assemble the membrane attack complex. This creates a hole or pore in the membrane that can kill or damage the pathogen or cell.
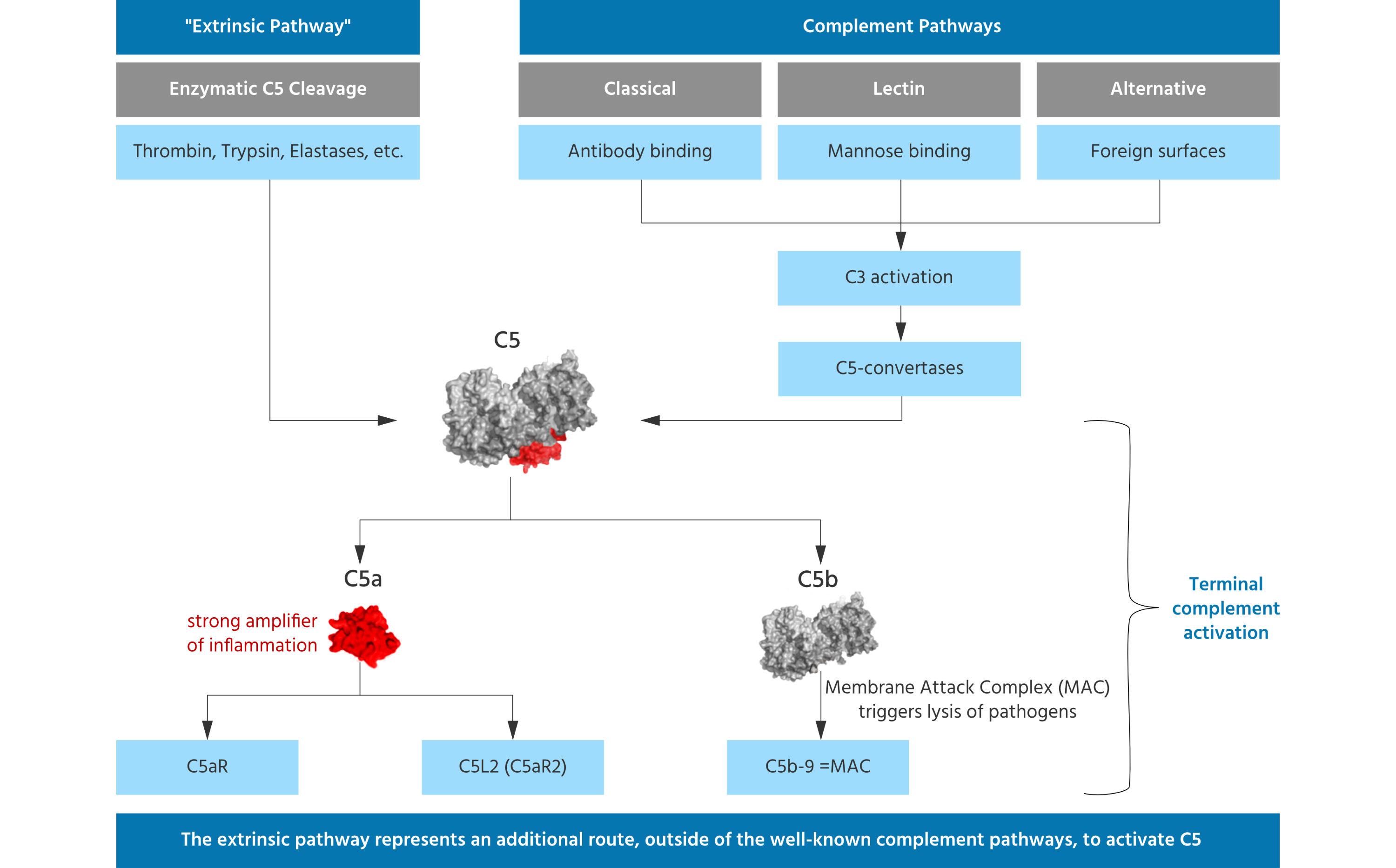
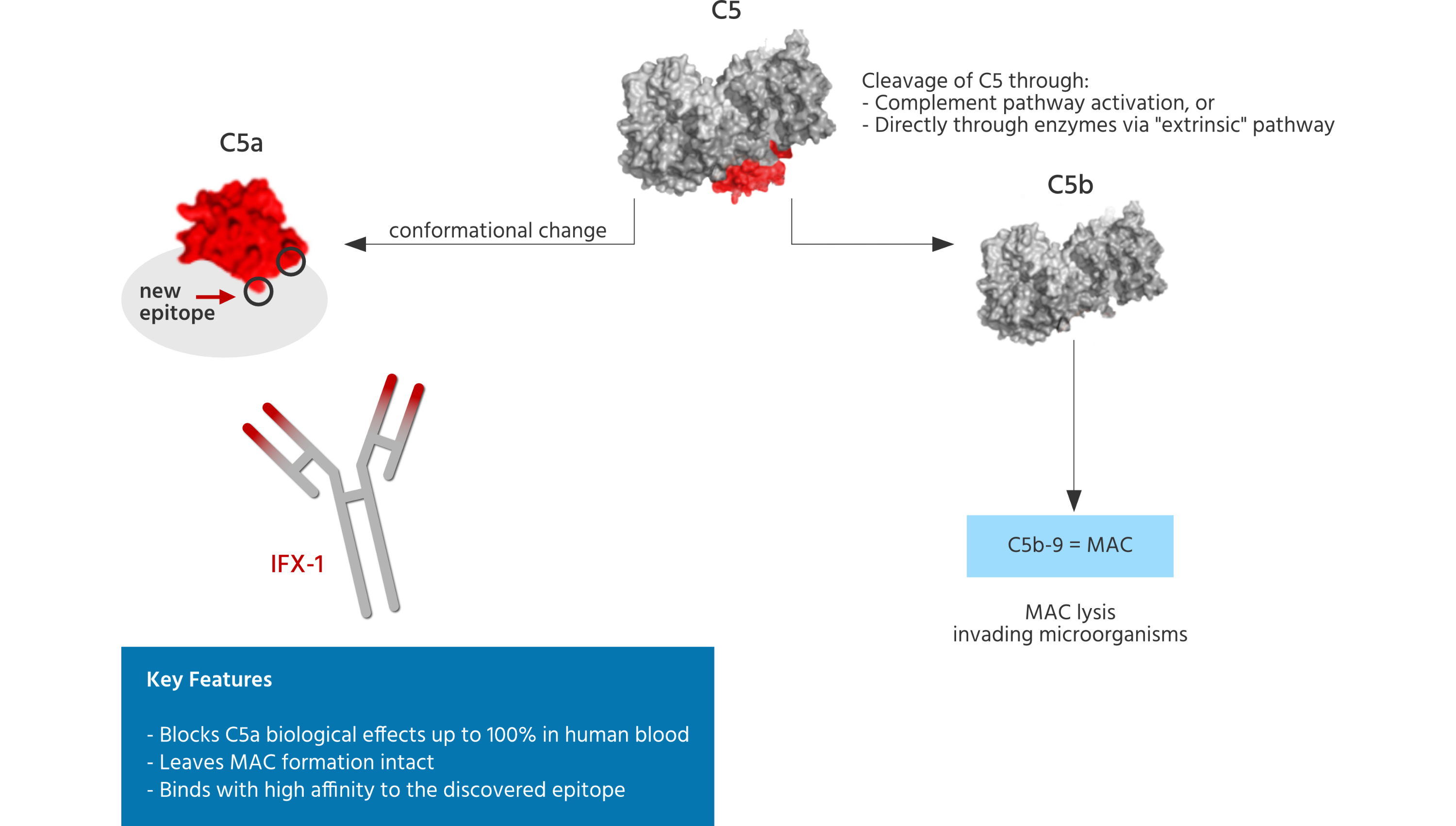
The cleavage of C5 is referred to as terminal complement activation, resulting in the very potent anaphylatoxin and "inflammatory booster" C5a and, on the other side, into C5b, which is part of the terminal membrane attack complex (referred to as MAC), which can permeabilize membranes of microorganisms, leading to their lysis. Thus, MAC formation is an important innate defense mechanism. Blocking MAC formation will lead to susceptibility to life threatening infections with encapsulated bacteria, such as meningococci, in humans.
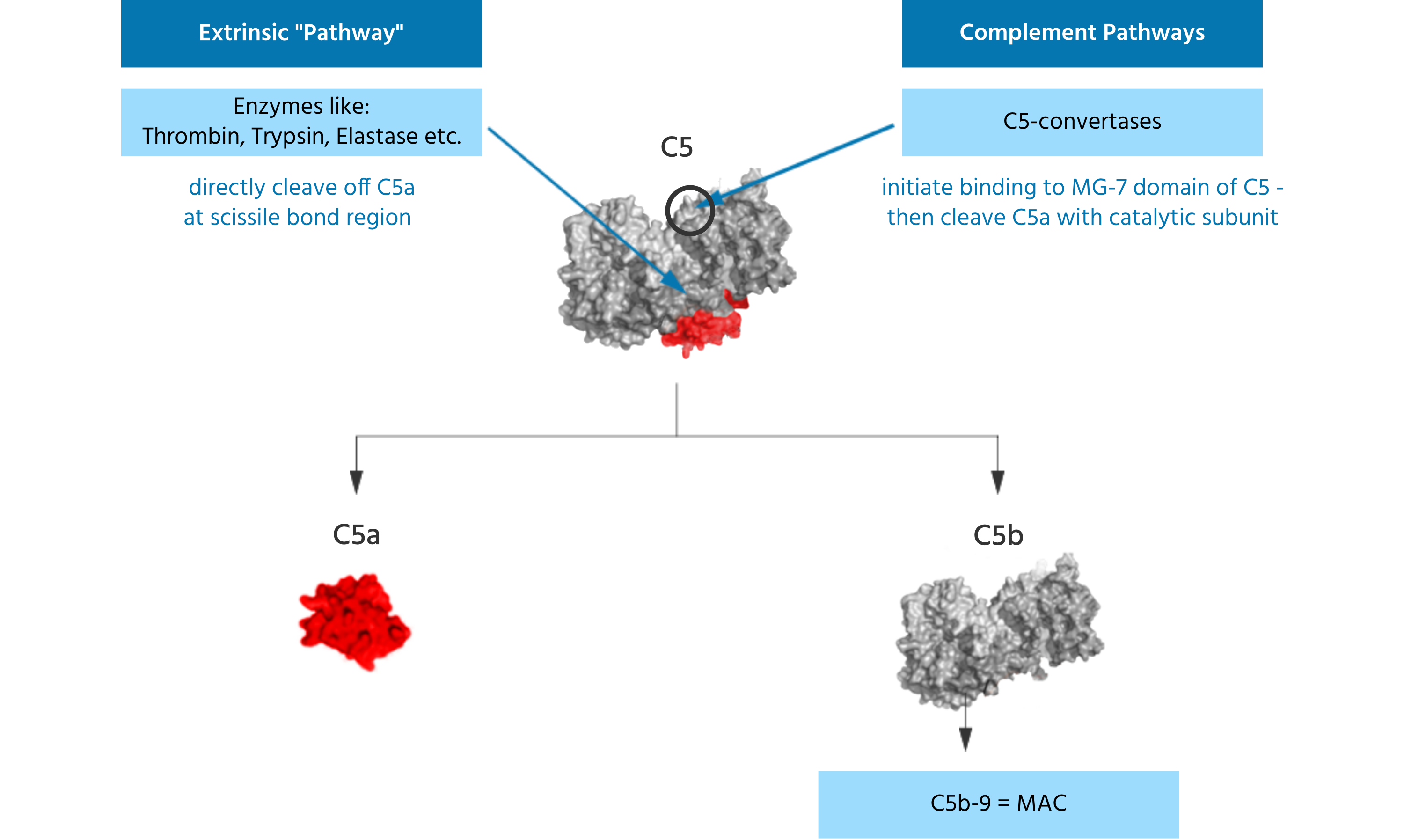
Noteworthy, terminal C5 activation does not require activation of the three complement pathways and related formation of the C5-convertases. Other enzymes present, e.g. in the coagulation pathway or secreted by phagocytic cells can also directly cleave / activate C5 such that C5a and C5b are formed. The C5 molecule has different binding and cleavage sites for enzymes to disengage C5a from the complex. Liver cells are known producers of C5 and contribute to blood C5 levels. However, various other epithelial cells have been found to also produce C5. It was shown that lung epithelial cells cultured in vitro can generate C5 upon stimulation and lung macrophages added to such cultures were able to cleave / activate C5 leading to generation of C5a in the absence of other complement factors in the cell culture. Thus, C5 can be activated and C5a can be generated independently from the complement pathways.
C5a is a small 74 amino acid spanning protein (approximately 11 KD) which is known to quickly interact with at least 2 independent receptors (C5aR and C5L2). C5aR has been well characterized as G-Protein coupled signaling receptor, which can be strongly upregulated in almost any tissue and cell, and in different disease settings. C5a therefore displays a short half-life, given the quick interaction with and the abundant number of the binding receptors.
The blood contains approximately 400 nM of C5, which is believed to increase under disease conditions. Thus, a large amount of C5a can be quickly activated from this C5 pool. C5 is not only generated in the liver but also in other epithelial cells like lung cells. Enzymes from phagocytes, have the capability of activating / cleaving C5. Thus, C5a can be generated without any involvement of the complement pathways.
In the acute inflammatory response, C5a holds a key player position, being involved as central accelerator or “booster” of inflammation. This role of C5a extents to a broad variety of responses: C5a robustly boosts the generation of many inflammatory cytokines such as IL-8, IL-6, IL-17, TNF-alpha and many more in a variety of cells as well as in the blood stream in disease settings. When C5a binds to its receptors on neutrophils, they are strongly activated and move to the source of damage or infection (a phenomenon called chemotaxis), generating oxygen radicals and activated granular enzymes, both of which are believed to be major contributors to cellular and tissue damage in the host. In addition, activated neutrophils have been described to induce the formation of extracellular neutrophil traps (NETs), a phenomenon also known as NETosis. NETs provide for a high local concentration of antimicrobial components and bind, disarm, and kill microbes extracellularly independent of phagocytic uptake. In addition to their antimicrobial properties, NETs may serve as a physical barrier that prevents further spread of the pathogens.
This central function and multiple mechanisms make C5a a powerful weapon which, when strongly activated, can promote self-damage ultimately leading to organ dysfunction and failure.
C5a is a key player in many acute and chronic inflammatory processes, making C5a a highly attractive pharmacological target.
Many research groups globally have demonstrated in different acute inflammatory animal models across various species that blocking the C5a / C5aR signaling axes leads to large outcome benefits, improved organ performance and strongly reduced harmful inflammatory responses.
In addition to the above described effects, C5a has been shown to be involved in many other pathophysiological mechanisms, especially on the cellular level: C5a is capable of inducing a complex change in the cell signaling cascade of immune competent cells, like dendritic cells or macrophages, which leads to an altered and often intensified signal transduction of other known signaling stimuli, such as Toll-like receptor signaling, thereby enhancing / “boosting” such secondary signals. C5a also affects T-cell responses and skews a so-called pro-inflammatory Th1-type response, leading to the generation of even more pro-inflammatory cytokines. In addition, C5a is capable of inducing adhesion molecule expression on endothelial cells, leading to more neutrophil adherence and migration and altered endothelial cell signaling with impact on cellular functions. C5a also directly induced the release of tissue factor from endothelial cells, which is believed to be the crucial initiation point of the so-called extrinsic coagulation pathways, leading to blood clotting and associated downstream effects. C5a also stimulates the lipoxygenase pathway of arachidonic acid metabolism, especially affecting neutrophils and macrophages. These and many other functions not depicted here make C5a a central player in the inflammatory response.
In the chronic inflammatory response, C5a has been described as a potential factor for disturbing a balanced T-cell response by down-regulating regulatory T-cells and promoting pro-inflammatory T-cell responses such as Th-1 and Th-17 responses. Blocking the C5a / C5aR signaling axes restores this balance, which makes C5a a very interesting drug target in the context of auto-immune and chronic inflammatory diseases which are associated with dysbalanced T-cell responses.
InflaRx has discovered and globally patented a new conformational epitope within the C5a molecule allowing for generation of highly selective and potent blocking monoclonal antibodies (including vilobelimab and IFX002) directed against this target. These antibodies exert a complete immunological blockade and inhibition of C5a induced effects, which is important because of the abundant presence and generation capacity of C5a in the human body, which once generated or up-regulated in various disease settings, is believed to interact rapidly with its receptors which are abundantly present on most cell types in the human body. Thus, a suitable anti-C5a antibody must be capable of limiting such interaction rapidly, capturing C5a before it can interact with its receptors and cause downstream pro-inflammatory signaling effects. Furthermore, antibodies against C5a must be highly specific to leave the formation of the MAC intact. Undesired inhibition of C5 through non-specific C5a binders could inhibit the ability for C5b cleavage and subsequent MAC formation and thus bear the risk of susceptibility to life threatening infections caused by encapsulated bacteria for patients being treated with such antibodies. The ability to inhibit C5a in a highly specific way translates into an improved safety profile for the patient.
Targeting the C5aR receptor poses an alternative strategy to inhibit signaling along the C5a/C5aR pathway. InflaRx is therefore also developing orally available inhibitors of C5aR. Two C5a receptors, C5aR (also known as C5aR1 or CD88) and C5aR2 (also known as C5L2 or GPR77), mediate the biological activities of C5a. Activation of C5aR has broadly acknowledged pro-inflammatory roles, while activation of C5aR2 remains controversial having both pro- and anti-inflammatory roles. In animal models of sepsis, anti-C5a treatment ameliorated the development of inflammatory responses and improved survival. In addition, experimental evidence suggests that blockade of C5aR signaling similarly improves survival in animals with sepsis. Finally, C5aR antagonists have shown excellent therapeutic effects in numerous models of inflammatory diseases involving complement activation.
Unlike its ligand C5a, C5aR can be pharmacologically inhibited by low molecular weight molecules. It is generally believed that blockade of C5a using antibodies offers a fast, complete, and safe way to control C5a-induced inflammation. The advantage of a low molecular weight compound inhibitor to C5aR is that it can be administered orally, thereby offering broad, long-term ease of administration to patients. Through proper clinical investigation of these small molecule C5aR antagonists in diseases induced by the activation of C5a/C5aR axis, the safety and efficacy of these agents can be established. InflaRx is also developing INF904, a product candidate that targets the C5aR receptor in indications in which oral delivery is the medically preferred route of administration.